| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |
| ||||||||||||||||||||
Access via PAY PER ARTICLE
|
|
|||||||||||||||||||||||||||||
Access via PAY PER ARTICLE |
||||||||||||||||||||||||||||||
Systematics and Phytogeography |
Department of Ecology and Evolutionary Biology, Yale University, P.O. Box 208106, New Haven, Connecticut 06520-8106 USA
Received for publication 23 December 2007. Accepted for publication 4 June 2008.
ABSTRACT
Interspecific hybridization followed by polyploidization appears to have played a major role in plant diversification, but quantifying the contribution of this mechanism to diversification within taxonomically complex clades remains difficult. Incongruence among gene trees can provide critical insights, especially when combined with data on chromosome numbers, morphology, and geography. To further test our previous hypothesis on hybrid speciation in Persicaria (Polygonaceae), we performed molecular phylogenetic studies using three cpDNA regions and nuclear ITS sequences, with an emphasis on sampling within section Eupersicaria. Our analyses revealed major conflicts between the combined cpDNA tree and the nrITS tree; a variety of incongruence tests rejected stochastic error as the cause of incongruence in most cases. On the basis of our tree incongruence results and information on chromosome numbers, we hypothesize that the origin of 10 polyploid species involved interspecific hybridization. Our studies also support the recognition of several previously named species that have been treated as belonging within other species. Repeated allotetraploidy (as distinct from radiation at the tetraploid level) now appears to be the key mechanism governing the diversification of this taxonomically challenging group.
Key Words: allopolyploidy • cpDNA • Eupersicaria • hybridization • incongruence test • ITS • Persicaria • Polygonaceae • Polygonum
Hybridization between separately evolving species, followed by polyploidization, has long been appreciated to be a key mechanism in plant evolution (e.g., Stebbins, 1950![]() ; Grant, 1981
; Grant, 1981![]() ; Arnold, 1997
; Arnold, 1997![]() , 2006
, 2006![]() ; Soltis and Soltis, 1999
; Soltis and Soltis, 1999![]() ; Abbott and Lowe, 2004
; Abbott and Lowe, 2004![]() ; Comai, 2005
; Comai, 2005![]() ; Paun et al., 2007
; Paun et al., 2007![]() ; Rieseberg and Willis, 2007
; Rieseberg and Willis, 2007![]() ). Because allopolyploidy can result in instant reproductive isolation, it has been viewed as driving diversification within some plant clades and especially as allowing new species to arise in sympatry. During the past two decades, molecular phylogenetic approaches have been brought to bear on this issue, and hybridization and allopolyploidy have now been carefully documented in a number of plant groups (e.g., Cardamine: Lihova et al., 2006
). Because allopolyploidy can result in instant reproductive isolation, it has been viewed as driving diversification within some plant clades and especially as allowing new species to arise in sympatry. During the past two decades, molecular phylogenetic approaches have been brought to bear on this issue, and hybridization and allopolyploidy have now been carefully documented in a number of plant groups (e.g., Cardamine: Lihova et al., 2006![]() ; Cerastium: Brysting et al., 2007
; Cerastium: Brysting et al., 2007![]() ; Glycine: Doyle et al., 2004
; Glycine: Doyle et al., 2004![]() ; Senecio: Abbott and Lowe, 2004
; Senecio: Abbott and Lowe, 2004![]() ; Tragopogon: Soltis et al., 2004
; Tragopogon: Soltis et al., 2004![]() ). However, quantifying the extent to which allopolyploidy has been a factor in the evolution of taxonomically complex groups in which it may have played a role remains difficult. In such groups we may both underestimate the number of species (cf. Soltis et al., 2007
). However, quantifying the extent to which allopolyploidy has been a factor in the evolution of taxonomically complex groups in which it may have played a role remains difficult. In such groups we may both underestimate the number of species (cf. Soltis et al., 2007![]() ) and misestimate the number of instances of ployploid speciation. On one end of the spectrum, the number of such events could equal (or even exceed, in the cases of multiple origins; see Soltis et al., 2003
) and misestimate the number of instances of ployploid speciation. On one end of the spectrum, the number of such events could equal (or even exceed, in the cases of multiple origins; see Soltis et al., 2003![]() ; Hegarty and Hiscock, 2005
; Hegarty and Hiscock, 2005![]() ) the number of polyploid species recognized in the group; that is, every polyploid species may have originated independently from diploid progenitors. At the other end, all the polyploid species in the group could have resulted from diversification at the polyploid level following just a single instance of allopolyploidy.
) the number of polyploid species recognized in the group; that is, every polyploid species may have originated independently from diploid progenitors. At the other end, all the polyploid species in the group could have resulted from diversification at the polyploid level following just a single instance of allopolyploidy.
Here we attempt to assess the nature and extent of allopolyploidy in a major clade within Persicaria (Polygonaceae) by comparing gene trees based on nuclear and chloroplast markers, and by considering these results in the context of information on chromosome numbers, morphology, and geography. The Eupersicaria clade has long been recognized as being taxonomically complex (described in next section), and it contains numerous polyploid species. Hybridization has been suggested as a factor contributing to the pronounced morphological variability in the group, and our previous studies (focused on the higher-level phylogeny of Persicaria; Kim and Donoghue, 2008![]() ) have highlighted the possibility of allopolyploidy within Eupersicaria. However, until now this possibility of allopolyploidy has not been critically examined using molecular techniques and an extensive sample of relevant species. Our studies also provide a critical application of a battery of tests to explore the nature and extent of incongruence between chloroplast and nuclear data sets.
) have highlighted the possibility of allopolyploidy within Eupersicaria. However, until now this possibility of allopolyploidy has not been critically examined using molecular techniques and an extensive sample of relevant species. Our studies also provide a critical application of a battery of tests to explore the nature and extent of incongruence between chloroplast and nuclear data sets.
Eupersicaria within Persicaria and Polygonaceae
Eupersicaria, composed of  70 species, was recognized as a section within Persicaria by Gross (1913)
70 species, was recognized as a section within Persicaria by Gross (1913)![]() . It includes species of Polygonum sections Persicaria and Amblygonum in the sense of Meisner (Meisner, 1826
. It includes species of Polygonum sections Persicaria and Amblygonum in the sense of Meisner (Meisner, 1826![]() , Meisner, 1856
, Meisner, 1856![]() ) or of Persicaria sect. Persicaria in the sense of Haraldson (1978
) or of Persicaria sect. Persicaria in the sense of Haraldson (1978![]() ). We use the name Eupersicaria for a clade identified by Kim and Donoghue (2008)
). We use the name Eupersicaria for a clade identified by Kim and Donoghue (2008)![]() that is nested within a more narrowly circumscribed Persicaria clade that corresponds to the genus Persicaria in the sense of Haraldson (1978)
that is nested within a more narrowly circumscribed Persicaria clade that corresponds to the genus Persicaria in the sense of Haraldson (1978)![]() . Within Persicaria, Eupersicaria is most closely related to two other named clades, Tovara and Echinocaulon, although the relationship among these three is not yet fully resolved (Lamb Frye and Kron, 2003
. Within Persicaria, Eupersicaria is most closely related to two other named clades, Tovara and Echinocaulon, although the relationship among these three is not yet fully resolved (Lamb Frye and Kron, 2003![]() ; Kim and Donoghue, 2008
; Kim and Donoghue, 2008![]() ). Here we do not assign formal ranks to these clades, but use Persicaria as the "genus" or "clade address" in referring to species of the Eupersicaria clade.
). Here we do not assign formal ranks to these clades, but use Persicaria as the "genus" or "clade address" in referring to species of the Eupersicaria clade.
Plants in Eupersicaria are easily distinguished from other groups by narrow lanceolate leaves and spike-like racemes with densely or loosely arranged flower fascicles. Most species in Eupersicaria have been delimited based on gross morphology relating to vegetative and reproductive characters such as leaf shape, trichome type and density, ochrea shape and marginal trichomes, perianth number, color and venation pattern, style number, and achene shape. In addition to morphological characters, growth form and habitat preferences have been used in species circumscription. Although these morphological and ecological characters are useful in recognizing some species, many of them are highly variable, resulting in great taxonomic confusion. For instance, Greene (1904)![]() described 38 new species, most of which are now generally considered to be part of the Persicaria amphibia complex (Mitchell, 1968
described 38 new species, most of which are now generally considered to be part of the Persicaria amphibia complex (Mitchell, 1968![]() ; Mitchell and Dean, 1978
; Mitchell and Dean, 1978![]() ; Hinds and Freeman, 2005
; Hinds and Freeman, 2005![]() ). Many infraspecific entities have been segregated within some polymorphic species such as P. lapathifolia, P. hydropiperoides, and P. punctata (Danser, 1921
). Many infraspecific entities have been segregated within some polymorphic species such as P. lapathifolia, P. hydropiperoides, and P. punctata (Danser, 1921![]() ; Stanford, 1926
; Stanford, 1926![]() ; Fassett, 1949
; Fassett, 1949![]() ; Fernald, 1950
; Fernald, 1950![]() ).
).
Owing to the high degree of morphological variation underlying such taxonomic confusion, some "species" of Eupersicaria have provided model systems for studies of phenotypic plasticity. Various moisture conditions relate to trichome, leaf, and ochrea variation in P. amphibia (Mitchell, 1968![]() , 1976
, 1976![]() ). Likewise, populations of P. maculosa grown under various conditions exhibit plasticity of ecological significance (Bell and Sultan, 1999
). Likewise, populations of P. maculosa grown under various conditions exhibit plasticity of ecological significance (Bell and Sultan, 1999![]() ; Sultan, 2001
; Sultan, 2001![]() , 2003
, 2003![]() ). Morphological variability in Eupersicaria has also been attributed to hybridization. Studies based on morphology (Stanford, 1925b
). Morphological variability in Eupersicaria has also been attributed to hybridization. Studies based on morphology (Stanford, 1925b![]() ; Timson, 1964
; Timson, 1964![]() ), experimental fertilization (Timson, 1964
), experimental fertilization (Timson, 1964![]() ; McDonald, 1980
; McDonald, 1980![]() ), and isozyme profiling (Consaul et al., 1991
), and isozyme profiling (Consaul et al., 1991![]() ) have highlighted hybridization as a factor contributing to variability in Eupersicaria.
) have highlighted hybridization as a factor contributing to variability in Eupersicaria.
Assessing incongruence between nuclear and chloroplast data sets
Besides stochastic error, genealogical discordances may be caused by horizontal gene transfer, hybridization, lineage sorting, or heterogeneous rates of molecular evolution (e.g., Maddison, 1997![]() ). To assess the statistical significance of tree incongruence, many tests have been proposed (see Goldman et al., 2000
). To assess the statistical significance of tree incongruence, many tests have been proposed (see Goldman et al., 2000![]() ; Planet, 2006
; Planet, 2006![]() , and references therein). Data congruence of separate matrices can initially be assessed by measuring the difference between the parsimony tree lengths inferred from randomly rearranged partitions and the tree length for the entire data set (e.g., incongruence length difference [ILD] test and its relatives; Farris et al., 1994
, and references therein). Data congruence of separate matrices can initially be assessed by measuring the difference between the parsimony tree lengths inferred from randomly rearranged partitions and the tree length for the entire data set (e.g., incongruence length difference [ILD] test and its relatives; Farris et al., 1994![]() , 1995
, 1995![]() ; Lecointre et al., 1998
; Lecointre et al., 1998![]() ; Thornton and DeSalle, 2000
; Thornton and DeSalle, 2000![]() ; Zelwer and Daubin, 2004
; Zelwer and Daubin, 2004![]() ). Although this matrix level approach has been criticized for its susceptibility to type I error (incorrectly rejecting the null hypothesis; Dolphin and Quicke, 2000
). Although this matrix level approach has been criticized for its susceptibility to type I error (incorrectly rejecting the null hypothesis; Dolphin and Quicke, 2000![]() ; Yoder et al., 2001
; Yoder et al., 2001![]() ; Dowton and Austin, 2002
; Dowton and Austin, 2002![]() ; Hipp et al., 2004
; Hipp et al., 2004![]() ), it is useful as a starting point because of the low type II error rate (accepting the incorrect null hypothesis) when a sufficient number of informative sites are included (Darlu and Lecointre, 2002
), it is useful as a starting point because of the low type II error rate (accepting the incorrect null hypothesis) when a sufficient number of informative sites are included (Darlu and Lecointre, 2002![]() ). So-called paired-sites tests (Felsenstein, 2004
). So-called paired-sites tests (Felsenstein, 2004![]() ), focused on specific conflicts, have been developed for parsimony (e.g., the Templeton test; Templeton, 1983
), focused on specific conflicts, have been developed for parsimony (e.g., the Templeton test; Templeton, 1983![]() ; Mason-Gamer and Kellogg, 1996
; Mason-Gamer and Kellogg, 1996![]() ) and likelihood (e.g., Kishino and Hasegawa [1989
) and likelihood (e.g., Kishino and Hasegawa [1989![]() ; K-H] and Shimodaira–Hasegawa [1999
; K-H] and Shimodaira–Hasegawa [1999![]() ;S-H] tests). Finally, the approximately unbiased (AU) test has been developed to reduce biases in the tests assuming smoothness of the boundaries of the hypothesis regions (Shimodaira, 2002
;S-H] tests). Finally, the approximately unbiased (AU) test has been developed to reduce biases in the tests assuming smoothness of the boundaries of the hypothesis regions (Shimodaira, 2002![]() ). The K-H, S-H, and AU methods use nonparametric bootstrapping, resampling estimated log-likelihoods (Kishino et al., 1990
). The K-H, S-H, and AU methods use nonparametric bootstrapping, resampling estimated log-likelihoods (Kishino et al., 1990![]() ) to generate a distribution for testing the hypothesis, whereas the Templeton test employs a Wilcoxon signed-rank test (Templeton, 1983
) to generate a distribution for testing the hypothesis, whereas the Templeton test employs a Wilcoxon signed-rank test (Templeton, 1983![]() ).
).
Although there are many methods to test the statistical significance of tree incongruence, we lack specific methods to distinguish among the underlying causes for incongruence between different data sets. Horizontal gene transfer, while common in bacteria and archaea (Doolittle et al., 2003![]() ), is fairly rare in plants, although examples have been reported recently for mitochondrial genes (see Richardson and Palmer, 2007
), is fairly rare in plants, although examples have been reported recently for mitochondrial genes (see Richardson and Palmer, 2007![]() , and references therein). Distinguishing between lineage sorting and hybridization is, in general, more difficult, although this distinction may be possible by taking into account branch lengths (Holder et al., 2001
, and references therein). Distinguishing between lineage sorting and hybridization is, in general, more difficult, although this distinction may be possible by taking into account branch lengths (Holder et al., 2001![]() ). In spite of the general difficulty in sorting out the causes, hybridization has often been favored when there are disparities between trees inferred from nuclear vs. chloroplast DNA sequences because chloroplasts are typically maternally inherited in angiosperms while nuclear genes are inherited from both parents. Hybridization has often been favored for polyploids (Arnold, 1997
). In spite of the general difficulty in sorting out the causes, hybridization has often been favored when there are disparities between trees inferred from nuclear vs. chloroplast DNA sequences because chloroplasts are typically maternally inherited in angiosperms while nuclear genes are inherited from both parents. Hybridization has often been favored for polyploids (Arnold, 1997![]() ; Rieseberg, 1997
; Rieseberg, 1997![]() ; Sang et al., 1997
; Sang et al., 1997![]() ; Soltis and Soltis, 1999
; Soltis and Soltis, 1999![]() ; Otto and Whitton, 2000
; Otto and Whitton, 2000![]() ; Doyle et al., 2004
; Doyle et al., 2004![]() ; Soltis et al., 2004
; Soltis et al., 2004![]() ). In general, the most convincing explanations combine data on morphology, chromosome number, and distribution patterns or ecology, with incongruence between nuclear and cpDNA trees.
). In general, the most convincing explanations combine data on morphology, chromosome number, and distribution patterns or ecology, with incongruence between nuclear and cpDNA trees.
MATERIALS AND METHODS
Taxon sampling
We included 63 accessions to represent 49 species: 40 species of Eupersicaria (54 accessions), four of Echinocaulon, and two of Tovara. Three species of the Cephalophilon clade were included for rooting purposes base on the results of Kim and Donoghue (2008)![]() . Appendix 1 provides voucher information on all accessions. Living samples were primarily collected by S.T.K. from fieldwork in China, Greece, Korea, and the United States during 2002–2005. Twenty-two populations from 19 species were sampled using herbarium specimens from the Harvard University Herbaria (GH), the University of New Hampshire Herbarium (NHA), and the Yale University Herbarium (YU). We included 17 accessions used in previous studies (Kim and Donoghue, 2008
. Appendix 1 provides voucher information on all accessions. Living samples were primarily collected by S.T.K. from fieldwork in China, Greece, Korea, and the United States during 2002–2005. Twenty-two populations from 19 species were sampled using herbarium specimens from the Harvard University Herbaria (GH), the University of New Hampshire Herbarium (NHA), and the Yale University Herbarium (YU). We included 17 accessions used in previous studies (Kim and Donoghue, 2008![]() : GenBank accession numbers starting with EF in Appendix 1). For phylogenetic analyses, we used a data matrix with 60 terminals. Three populations of Persicaria punctata (P3–P5) were examined only in our exploration of nrITS polymorphism.
: GenBank accession numbers starting with EF in Appendix 1). For phylogenetic analyses, we used a data matrix with 60 terminals. Three populations of Persicaria punctata (P3–P5) were examined only in our exploration of nrITS polymorphism.
DNA extraction, PCR, and sequencing
Genomic DNA was extracted from fresh or dried leaf samples using a DNeasy Plant Mini Kit (Qiagen Valencia, California, USA). Because DNA extraction from relatively old herbarium specimens was inefficient compared to that from leaf samples dried in silica gel, we added a 20-h rocking incubation with proteinase K and 2-mercaptoethanol to the first step of the supplied protocol. Amplification of double-stranded DNA was carried out using standard polymerase chain reaction (PCR) in 25-µL reactions containing 1–10 ng DNA, 1.0 unit of Taq polymerase (Qiagen), 2.5 µL 10x buffer, 5 µL Q solution, and 1 µL MgCl2 to make final concentrations of 2.5 mmol/L, 1.0 mmol/L dNTPs (NEB, Ipswich, Massachusetts, USA), and 1.0 µmol/L amplification primers. The following primers were used: ITSLeu (Baum et al., 1998![]() ) and ITS4 (White et al., 1990
) and ITS4 (White et al., 1990![]() ) for the internal transcribed spacer region, including the 5.8S rRNA coding region (nrITS); matK-PA1F (Kim and Donoghue, 2008
) for the internal transcribed spacer region, including the 5.8S rRNA coding region (nrITS); matK-PA1F (Kim and Donoghue, 2008![]() ) and trnK2621 (Young et al., 1999
) and trnK2621 (Young et al., 1999![]() ) for the 5' trnK intron and partial matK region (p-matK); psbAF and trnHR for the psbA-trnH IGS (psbA; Sang et al., 1997
) for the 5' trnK intron and partial matK region (p-matK); psbAF and trnHR for the psbA-trnH IGS (psbA; Sang et al., 1997![]() ); and "c" and "f" for the IGS between trnL and trnF and the trnF intron (trnL-F; Taberlet et al., 1991
); and "c" and "f" for the IGS between trnL and trnF and the trnF intron (trnL-F; Taberlet et al., 1991![]() ). PCR cycles followed those in Kim and Donoghue (2008)
). PCR cycles followed those in Kim and Donoghue (2008)![]() . When gene regions were difficult to amplify from herbarium extractions, smaller fragments were amplified with internal sequencing primers (described later) using the conditions described. Because the amplification of the smaller fragments obtained from some species did not yield enough product for direct sequencing, we cloned the PCR products using a TOPO TA cloning kit (Invitrogen, Carlsbad, California, USA) following the supplied protocol. PCR products of nrITS from five accessions of P. punctata were cloned to examine haplotype polymorphism; more than 36 colonies were picked for each accession and sequenced.
. When gene regions were difficult to amplify from herbarium extractions, smaller fragments were amplified with internal sequencing primers (described later) using the conditions described. Because the amplification of the smaller fragments obtained from some species did not yield enough product for direct sequencing, we cloned the PCR products using a TOPO TA cloning kit (Invitrogen, Carlsbad, California, USA) following the supplied protocol. PCR products of nrITS from five accessions of P. punctata were cloned to examine haplotype polymorphism; more than 36 colonies were picked for each accession and sequenced.
PCR products were purified using a QIAquick PCR Purification Kit (Qiagen) or the polyethylene glycol–NaCl precipitation method (Lis and Schleif, 1975![]() ). Sequencing was carried out using the amplification primers and additional internal primers, as follows: ITS2 (White et al., 1990
). Sequencing was carried out using the amplification primers and additional internal primers, as follows: ITS2 (White et al., 1990![]() ) and ITS3b (the reverse sequence of ITS2) for the nrITS region, and "d" and "e" (Taberlet et al., 1991
) and ITS3b (the reverse sequence of ITS2) for the nrITS region, and "d" and "e" (Taberlet et al., 1991![]() ) for trnL-F. Cycle sequencing followed the protocol provided with the ABI PRISM Dye Primer Cycle Sequencing Ready Reaction Kit (Revision B, August 1995, Perkin-Elmer, Foster City, California, USA) and was visualized using a BaseStation 510 (MJ Research, Sauk City, Wisconsin, USA), an ABI 377, or an ABI 3100 automated DNA sequencer. Sequencing was conducted in part at the W. M. Keck Facility and the Science Hill DNA Analysis Facility at Yale University.
) for trnL-F. Cycle sequencing followed the protocol provided with the ABI PRISM Dye Primer Cycle Sequencing Ready Reaction Kit (Revision B, August 1995, Perkin-Elmer, Foster City, California, USA) and was visualized using a BaseStation 510 (MJ Research, Sauk City, Wisconsin, USA), an ABI 377, or an ABI 3100 automated DNA sequencer. Sequencing was conducted in part at the W. M. Keck Facility and the Science Hill DNA Analysis Facility at Yale University.
Alignments and phylogenetic analyses
Sequences were aligned using the program CLUSTAL_X (Thompson et al., 1997![]() ), T-coffee version 1.35 (Notredame, Higgins, and Heringa, 2000
), T-coffee version 1.35 (Notredame, Higgins, and Heringa, 2000![]() ), or MUSCLE version 3.6 (Edgar, 2004
), or MUSCLE version 3.6 (Edgar, 2004![]() ) and adjusted by eye to resolve minor conflicts. Our aligned data matrices and the trees published here are available in TreeBASE (SN3727, http://www.treebase.org) or upon request from the first author. Phylogenetic analyses were conducted using a nrITS data set and a combined data set of three chloroplast gene regions using PAUP* version 4.0b10 (Swofford, 2002
) and adjusted by eye to resolve minor conflicts. Our aligned data matrices and the trees published here are available in TreeBASE (SN3727, http://www.treebase.org) or upon request from the first author. Phylogenetic analyses were conducted using a nrITS data set and a combined data set of three chloroplast gene regions using PAUP* version 4.0b10 (Swofford, 2002![]() ) and MrBayes version 3.1 (Huelsenbeck and Ronquist, 2001
) and MrBayes version 3.1 (Huelsenbeck and Ronquist, 2001![]() ). Maximum parsimony (MP) searches were performed using heuristic search methods with tree-bisection-reconnection (TBR) branch swapping, collapse of zero maximum branch lengths, MULTREES option in effect, and equal weighting of all characters. Analyses were repeated 500 times with a random order of sequence addition in an attempt to sample multiple islands of most parsimonious trees. Bootstrap tests (Felsenstein, 1985
). Maximum parsimony (MP) searches were performed using heuristic search methods with tree-bisection-reconnection (TBR) branch swapping, collapse of zero maximum branch lengths, MULTREES option in effect, and equal weighting of all characters. Analyses were repeated 500 times with a random order of sequence addition in an attempt to sample multiple islands of most parsimonious trees. Bootstrap tests (Felsenstein, 1985![]() ) were carried out to evaluate node support using 1000 replicates. Heuristic search settings were identical to those for the original search for the combined cpDNA data set, whereas the MULTREES option was not in effect for the nrITS data set to avoid inappropriate rearrangements (Debry and Olmstead, 2000
) were carried out to evaluate node support using 1000 replicates. Heuristic search settings were identical to those for the original search for the combined cpDNA data set, whereas the MULTREES option was not in effect for the nrITS data set to avoid inappropriate rearrangements (Debry and Olmstead, 2000![]() ). We determined the best-fit model of sequence evolution in a series of hierarchical likelihood ratio tests (hLRT) using MODELTEST version 3.7 (Posada and Crandall, 1998
). We determined the best-fit model of sequence evolution in a series of hierarchical likelihood ratio tests (hLRT) using MODELTEST version 3.7 (Posada and Crandall, 1998![]() ). Maximum likelihood (ML) searches were carried out in PAUP* using the models selected by hLRT for each data set (Appendix S1, see Supplemental Data with online version of this article.). Parameters for each search were simultaneously estimated via maximum likelihood for all datasets. Heuristic search methods were used with TBR branch swapping and collapse of zero-length branches. Analyses were repeated 100 times with a random order of sequence addition. Bootstrap tests were performed using 500 replicates with nearest neighbor interchange (NNI) branch swapping. Parameters for bootstrap tests were fixed to values estimated from the maximum likelihood tree. Bayesian inferences were conducted using the models selected using MODELTEST. Also, for the combined data set of three chloroplast gene regions, we applied different models selected from MODELTEST for each partition. Five million generations were run to estimate parameters relating to sequence evolution and likelihood probabilities using a Markov chain Monte Carlo (MCMC) method. Trees were collected every 100th generation. After removing 25% of the generations (125000 generations) as burn in, a 50% majority rule consensus tree was calculated to generate a posterior probability for each node.
). Maximum likelihood (ML) searches were carried out in PAUP* using the models selected by hLRT for each data set (Appendix S1, see Supplemental Data with online version of this article.). Parameters for each search were simultaneously estimated via maximum likelihood for all datasets. Heuristic search methods were used with TBR branch swapping and collapse of zero-length branches. Analyses were repeated 100 times with a random order of sequence addition. Bootstrap tests were performed using 500 replicates with nearest neighbor interchange (NNI) branch swapping. Parameters for bootstrap tests were fixed to values estimated from the maximum likelihood tree. Bayesian inferences were conducted using the models selected using MODELTEST. Also, for the combined data set of three chloroplast gene regions, we applied different models selected from MODELTEST for each partition. Five million generations were run to estimate parameters relating to sequence evolution and likelihood probabilities using a Markov chain Monte Carlo (MCMC) method. Trees were collected every 100th generation. After removing 25% of the generations (125000 generations) as burn in, a 50% majority rule consensus tree was calculated to generate a posterior probability for each node.
Incongruence tests
Tree incongruence was assessed using the five different approaches noted in the introduction. (1) The ILD test was performed using the partition homogeneity test implemented in PAUP* with simple taxon addition, TBR branch swapping, and heuristic searches of 1000 repartitions of the data. Pairwise tests between each data set and between the combined cpDNA and nrITS data sets were carried out to assess how much the original partitions differed from random partitions in parsimony tree length. (2) Templeton tests were performed using PAUP* to assess the contribution of specific nodes to the conflict between trees. A "test tree," the strict consensus of the most parsimonious trees inferred from a given data set, was compared to two types of "rival trees": (1) the strict consensus of the most parsimonious trees inferred from another data set and (2) modified "test trees" with constrained nodes where topological conflict was observed ("test" and "rival" are used here as in the sense of Mason-Gamer and Kellogg, 1996![]() ). For example, where a particular conflict in tree topology existed between the strict consensus trees from nrITS and the combined cpDNA data sets, we specifically modified the nrITS tree to reflect each conflicting relationship suggested by the cpDNA tree and then compared the nrITS strict consensus tree (test tree) to each modified trees (rival tree) for the nrITS data set. Likewise, we tested rival trees suggested by the nrITS using the cpDNA data set. The last three tests—(3) the K-H test, (4) the S-H test, and (5) the AU test—used the likelihood-based
). For example, where a particular conflict in tree topology existed between the strict consensus trees from nrITS and the combined cpDNA data sets, we specifically modified the nrITS tree to reflect each conflicting relationship suggested by the cpDNA tree and then compared the nrITS strict consensus tree (test tree) to each modified trees (rival tree) for the nrITS data set. Likewise, we tested rival trees suggested by the nrITS using the cpDNA data set. The last three tests—(3) the K-H test, (4) the S-H test, and (5) the AU test—used the likelihood-based 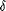 statistic to test competing hypotheses postulated by different tree topologies. The null distribution was generated by nonparametric boostrapping using the RELL method (Kishino and Hasegawa, 1989
statistic to test competing hypotheses postulated by different tree topologies. The null distribution was generated by nonparametric boostrapping using the RELL method (Kishino and Hasegawa, 1989![]() ; Kishino et al., 1990
; Kishino et al., 1990![]() ). We calculated log likelihood scores of trees constrained by topological conflicts using PAUP* and test values including P values using the program CONSEL (Shimodaira and Hasegawa, 2001
). We calculated log likelihood scores of trees constrained by topological conflicts using PAUP* and test values including P values using the program CONSEL (Shimodaira and Hasegawa, 2001![]() ).
).
RESULTS
Aligned DNA sequences
Information on the sequences, including total sequence length and % GC content for our four gene regions are presented in Appendix S1. Sequence lengths varied in the noncoding regions of each gene region, and indels were introduced for alignment. The aligned sequence length for the nrITS region was 687 bp and for the combined cpDNA regions (cp-combined) was 2023 bp. The cp-combined data set contained more parsimony-informative sites (PIS) than nrITS, but showed slightly less variability in the ratio of PIS to variable sites (Appendix S1, see Supplemental Data with online version of article). We did not include indels in our phylogenetic analyses because most data sets contained inconsistent, variously overlapped indels of questionable phylogenetic significance. There were 21-bp short inversions attaching inverted repeats of 17 bp (complementary reversed regions) in psbA sequences, as reported by Kim and Donoghue (2008)![]() for five species belonging to Cephalophilon and Echinocaulon. Three additional species of Eupersicaria, Persicaria densiflora, P. hydropiperoides.P2, and P. kawagoeana had the same inversions in psbA. These fragments were inverted for alignment and included in our phylogenetic analyes as in Kim and Donoghue (2008)
for five species belonging to Cephalophilon and Echinocaulon. Three additional species of Eupersicaria, Persicaria densiflora, P. hydropiperoides.P2, and P. kawagoeana had the same inversions in psbA. These fragments were inverted for alignment and included in our phylogenetic analyes as in Kim and Donoghue (2008)![]() . We also conducted phylogenetic analyses without these regions; the same tree topologies were recovered with only slight differences in confidence values (data not shown).
. We also conducted phylogenetic analyses without these regions; the same tree topologies were recovered with only slight differences in confidence values (data not shown).
Phylogenetic analysis of the combined cpDNA data set
Our phylogenetic analyses of cpDNA focused on the combined data set as pairwise ILD tests indicated that the three cpDNA regions were not significantly different from one another (Table 1). The strict consensus tree from 416 most parsimonious trees (tree length [TL] = 48; consistency index [CI] = 0.867; retention index [RI] = 0.938) and the maximum likelihood tree from heuristic searches (model selected = K81uf + G; –log likelihood = 5921.876) are presented in Fig. 1A, and the 50% majority rule consensus tree from Bayesian inference (model selected for each data set: psbA = F81 +G, p-matK = K81uf + G, trnLF = F81 + G; mean –log likelihood = 6585.224) is presented in Fig. 2A. The monophyly of Eupersicaria is strongly supported in MP and ML analyses with 100% bootstrap (BP) support and 1.00 posterior probability (PP; Figs. 1 and 2). Our cpDNA combined data analyses show strong support for P. amphibia as sister to the rest of Eupersicaria.
|
|
|
 P. japonica ("
P. japonica (" " signifies the inclusion of all species between the two on our trees); (2) P. hydropiperoides.P2
" signifies the inclusion of all species between the two on our trees); (2) P. hydropiperoides.P2  P. viscofera; and (3) P. bicornis
P. viscofera; and (3) P. bicornis  P. densiflora. Within the first clade in Fig. 1A, two strongly supported clades and one with weaker support (stronger in Bayesian analysis, Fig. 2A) were suggested, although relationships among these were not well resolved in any analysis. Persicaria kawagoeana and P. barbata form a clade with strong supports in all analyses. Interspecific relationships within the other strongly supported clade (P. macrantha
P. densiflora. Within the first clade in Fig. 1A, two strongly supported clades and one with weaker support (stronger in Bayesian analysis, Fig. 2A) were suggested, although relationships among these were not well resolved in any analysis. Persicaria kawagoeana and P. barbata form a clade with strong supports in all analyses. Interspecific relationships within the other strongly supported clade (P. macrantha  P. maculosa; Fig. 1A) were ambiguous in both MP and ML analyses but well resolved in the Bayesian analysis (Fig. 2A). In contrast, relationships within the weakly supported clade (P. hydropiper.P1
P. maculosa; Fig. 1A) were ambiguous in both MP and ML analyses but well resolved in the Bayesian analysis (Fig. 2A). In contrast, relationships within the weakly supported clade (P. hydropiper.P1  P. minor.P2; Fig. 1A) were well resolved, with P. hydropiper and P. pubescens forming a clade. The second major clade (P. hydropiperoides.P2
P. minor.P2; Fig. 1A) were well resolved, with P. hydropiper and P. pubescens forming a clade. The second major clade (P. hydropiperoides.P2  P. viscofera; Fig. 1A) was mostly composed of American species except the East Asian P. viscofera. Relationships within this clade were resolved with moderate to weak support in MP and ML analyses, but with relatively strong support in the Bayesian analysis (Figs. 1A and 2A). Our three accessions of P. hydropiperoides did not appear together. One formed a clade with P. setaceae and P. hirsuta and another with P. puritanorum and P. punctata.P2; the position of the third accession remained unresolved (Figs. 1A and 2A). Persicaria viscofera appears to be sister to the remaining species in the second major clade (Figs. 1A and 2A).
P. viscofera; Fig. 1A) was mostly composed of American species except the East Asian P. viscofera. Relationships within this clade were resolved with moderate to weak support in MP and ML analyses, but with relatively strong support in the Bayesian analysis (Figs. 1A and 2A). Our three accessions of P. hydropiperoides did not appear together. One formed a clade with P. setaceae and P. hirsuta and another with P. puritanorum and P. punctata.P2; the position of the third accession remained unresolved (Figs. 1A and 2A). Persicaria viscofera appears to be sister to the remaining species in the second major clade (Figs. 1A and 2A).
Relationships within the third major clade revealed in every analysis (P. bicornis  P. densiflora) were not well resolved except for several clades with moderate confidence: P. bicornis with P. mexicana, P. glabra.P2 with P. hispida, and P. tomentosa with P. senegalensis (Figs. 1A and 2A). Parsimony analyses also suggested an unresolved clade including P. tomentosa
P. densiflora) were not well resolved except for several clades with moderate confidence: P. bicornis with P. mexicana, P. glabra.P2 with P. hispida, and P. tomentosa with P. senegalensis (Figs. 1A and 2A). Parsimony analyses also suggested an unresolved clade including P. tomentosa  P. densiflora, but with less than 50% bootstrap support. Likelihood analysis resolved more relationships within this clade but again with bootstrap values below 50% (Fig. 1A). In contrast, some relationships in the Bayesian analysis were well supported (Fig. 2A).
P. densiflora, but with less than 50% bootstrap support. Likelihood analysis resolved more relationships within this clade but again with bootstrap values below 50% (Fig. 1A). In contrast, some relationships in the Bayesian analysis were well supported (Fig. 2A).
The clade including P. limbata and P. accuminata (MP/ML/PP = 80/90/1.00), and P. careyi, were linked as sister to the three major clades discussed in parsimony and Bayesian analyses. Likelihood analysis suggested a close relationship of P. careyi to the clade including P. limbata and P. accuminata, but with less than 50% bootstrap support (Fig. 1A).
Phylogenetic analysis of the nrITS data set
Information on our phylogenetic analyses using nrITS data are summarized in Appendix S1. A strict consensus tree from 305 most parsimonious trees (TL = 594; CI = 0.647; RI = 0.827) and the ML tree from heuristic search (model selected = TrN + G; –log likelihood = 4200.498) are presented in Fig. 1B, and the 50% majority rule consensus tree from the Bayesian inference (model selected = TrN + G; mean –log likelihood = 4290.123) is shown in Fig. 2B. The monophyly of all Eupersicaria except for P. amphibia was strongly supported in all three analyses (MP/ML/PP = 100/100/1.00). However, basal relationships inferred from the nrITS data set differ from those in our cpDNA trees. The close relation of P. amphibia to the two representatives of Tovara (P. filiformis and P. virginiana) was consistent, despite low support values (MP/ML/PP = <50/<50/0.81). As in Kim and Donoghue (2008)![]() , relationships among the sectional level clades are not confidently resolved. Echinocaulon (P. arifolia, P. maackiana, P. sagittata, and P. meisneriana) is seen to be more closely related to Eupersicaria excluding P. amphibia (Figs. 1B and 2B) in ML and Bayesian analyses, but with weak support (ML/PP = <50%/0.53), whereas Echinocaulon appears as sister to the clade including Tovara and P. amphibia in MP analyses, again with a weak bootstrap support (54%; Fig. 1B).
, relationships among the sectional level clades are not confidently resolved. Echinocaulon (P. arifolia, P. maackiana, P. sagittata, and P. meisneriana) is seen to be more closely related to Eupersicaria excluding P. amphibia (Figs. 1B and 2B) in ML and Bayesian analyses, but with weak support (ML/PP = <50%/0.53), whereas Echinocaulon appears as sister to the clade including Tovara and P. amphibia in MP analyses, again with a weak bootstrap support (54%; Fig. 1B).
All three analyses using nrITS revealed seven major clades within Eupersicaria excluding P. amphibia, of which five were strongly supported and two were weakly supported (Figs. 1B and 2B). Relationships among these clades were not resolved in MP analyses except that the P. lapathifolia.P3  P. tomentosa clade is weakly supported (58% BP; Fig. 1B) as sister to the other six clades and three other species (P. japonica, P. viscosa, and P. careyi). Relationships among these clades were resolved in ML and Bayesian analyses, although confidence values in the ML analysis were below 50% (Fig. 1B). The strongly supported clade P. hydropiper.P1
P. tomentosa clade is weakly supported (58% BP; Fig. 1B) as sister to the other six clades and three other species (P. japonica, P. viscosa, and P. careyi). Relationships among these clades were resolved in ML and Bayesian analyses, although confidence values in the ML analysis were below 50% (Fig. 1B). The strongly supported clade P. hydropiper.P1  P. punctata.P2 (Fig. 1B) appears as sister to the P. macrantha
P. punctata.P2 (Fig. 1B) appears as sister to the P. macrantha  P. tinctoria clade with weak to moderate support (MP/ML/PP = 58/59/0.9). However, relationships within these two clades were not resolved except for a weakly supported relationship between P. maculosa and P. tinctoria (Figs. 1B and 2B). Nuclear ITS analyses suggest rather different placements of P. kawagoenan and P. punctata compare to our cpDNA analyses. Also, nrITS analyses suggest that P. foliosa and P. taquetii, seen as close relatives of P. macrantha and P. posumbu in the cpDNA analyses, are linked as sister group to P. macrantha
P. tinctoria clade with weak to moderate support (MP/ML/PP = 58/59/0.9). However, relationships within these two clades were not resolved except for a weakly supported relationship between P. maculosa and P. tinctoria (Figs. 1B and 2B). Nuclear ITS analyses suggest rather different placements of P. kawagoenan and P. punctata compare to our cpDNA analyses. Also, nrITS analyses suggest that P. foliosa and P. taquetii, seen as close relatives of P. macrantha and P. posumbu in the cpDNA analyses, are linked as sister group to P. macrantha  P. punctata.P2 (Fig. 1B). Within another strongly supported clade, P. puritanorum
P. punctata.P2 (Fig. 1B). Within another strongly supported clade, P. puritanorum  P. minor.P2 (MP/ML/PP = 98/96/1.00), the clade including P. hydropiperoides, P. puritanorum, and P. opelousana is most closely related to P. densiflora, and, in turn, to two accessions of P. minor (Fig. 1B). In marked contrast, P. densiflora joined P. lapathifolia and its relatives in our cpDNA analyses, and P. minor linked with P. hydropiper and P. pubescens (Fig. 1A).
P. minor.P2 (MP/ML/PP = 98/96/1.00), the clade including P. hydropiperoides, P. puritanorum, and P. opelousana is most closely related to P. densiflora, and, in turn, to two accessions of P. minor (Fig. 1B). In marked contrast, P. densiflora joined P. lapathifolia and its relatives in our cpDNA analyses, and P. minor linked with P. hydropiper and P. pubescens (Fig. 1A).
The P. mexicana  P. robustior clade includes an interesting combination of taxa and relationships in comparison with our cpDNA analyses. One nrITS group, consisting of P. hirsuta, P. setacea, and P. robustior, appears to be closely related to P. hydropiperoides and P. punctata in our cpDNA trees, whereas the later are spread apart in our nrITS trees (Figs. 1B and 2B). Moreover, the P. mexicana
P. robustior clade includes an interesting combination of taxa and relationships in comparison with our cpDNA analyses. One nrITS group, consisting of P. hirsuta, P. setacea, and P. robustior, appears to be closely related to P. hydropiperoides and P. punctata in our cpDNA trees, whereas the later are spread apart in our nrITS trees (Figs. 1B and 2B). Moreover, the P. mexicana  P. bicornis clade (MP/ML/PP = 100/100/1.00; Figs. 1B and 2B) includes species that do not appear to be at all closely related based on cpDNA.
P. bicornis clade (MP/ML/PP = 100/100/1.00; Figs. 1B and 2B) includes species that do not appear to be at all closely related based on cpDNA.
Our nrITS results also provided quite a different view of specific relationships within the P. accuminata  P. glabra.P2 clade. Although weakly supported in nrITS analyses, the monophyly of P. accuminata and P. limbata does correspond to the cpDNA analysis. However, these taxa are seen to be more closely related to P. orientalis in the nrITS analyses. The monophyly of P. hispida, P. paraguayensis, and P. glabra.P2 is strongly supported by nrITS, but the relationship of P. hispida directly to P. paraguayensis differs from the cpDNA for which P. hispida is more closely related to P. glabra.P2 (Figs. 1A and B).
P. glabra.P2 clade. Although weakly supported in nrITS analyses, the monophyly of P. accuminata and P. limbata does correspond to the cpDNA analysis. However, these taxa are seen to be more closely related to P. orientalis in the nrITS analyses. The monophyly of P. hispida, P. paraguayensis, and P. glabra.P2 is strongly supported by nrITS, but the relationship of P. hispida directly to P. paraguayensis differs from the cpDNA for which P. hispida is more closely related to P. glabra.P2 (Figs. 1A and B).
The monophyly of seven accessions (P. lapathifolia.P3  P. tomentosa; Figs. 1B and 2B) is supported in nrITS analyses with 100% BP and 1.00 PP. These are all part of the P. tomentosa
P. tomentosa; Figs. 1B and 2B) is supported in nrITS analyses with 100% BP and 1.00 PP. These are all part of the P. tomentosa  P. densiflora clade in the cpDNA tree (Fig. 1A), but relationships among them differ in the two trees. South American P. ferruginea and P. glabra.P1 are strongly clustered and more closely related to the clade including P. senegalensis and P. lapathifolia.P3 in our nrITS trees (Figs. 1B and 2B).
P. densiflora clade in the cpDNA tree (Fig. 1A), but relationships among them differ in the two trees. South American P. ferruginea and P. glabra.P1 are strongly clustered and more closely related to the clade including P. senegalensis and P. lapathifolia.P3 in our nrITS trees (Figs. 1B and 2B).
ML analysis suggest that the P. lapathifolia.P3  P. tomentosa clade is sister to the entire P. macranth
P. tomentosa clade is sister to the entire P. macranth  P. viscosa clade (Fig. 1B) and that the clade consisting of P. nodosa and P. viscofera is sister to all other core Eupersicaria (i.e., excluding P. amphibia). However, these results are only weakly supported.
P. viscosa clade (Fig. 1B) and that the clade consisting of P. nodosa and P. viscofera is sister to all other core Eupersicaria (i.e., excluding P. amphibia). However, these results are only weakly supported.
Incongruence tests
Our molecular analyses using nrITS and a combined cpDNA data set indicate many topological conflicts, some of which appear to be quite strong judging by support values. We conducted a battery of incongruence tests to evaluate the significance of these conflicts. Results from our Templeton tests are summarized in Table 2. Most constraints, in both directions, were rejected with >95% confidence, indicating that those differences are most likely not the result of stochastic error—8 of 13 cpDNA clades are rejected by the nrITS data, and 15 of 20 of the nrITS clades are rejected by cpDNA. Templeton tests indicate that the differing placements of P. foliosa and P. taquetii, and whether P. senegalensis is more closely related to P. tomentosa or to P. lapathifolia.P3, are not significant (constraints A, c-1, and I; Table 2, Fig. 1). Conflicts associated with the relationship among P. amphibia, Echinocaulon, and Tovara were not rejected by nrITS using cpDNA constraints, but were rejected by the cpDNA tree using nrITS constraints (constraints L, M, and q, r; Table 2, Fig. 1). Clades i and m from the nrITS tree are not significantly rejected by cpDNA (Table 2).
|
|
 P. glabra.P2, while these were rejected in the AU test but not in the K-H test. The S-H test showed very conservative behavior, as only 6 of 21 constraints were rejected, compared to the rejection of all constraints by the AU test, and 19 of 21 constraints by the K-H test (Table 3). Most of the conflicts, involving 23 species of the 40 species examined, appear to be statistically significant in both parsimony and likelihood-based tests (Tables 2 and 3). Interestingly, results of the S-H tests differed in suggesting fewer strong conflicts. The S-H test was proposed to compensate for the K-H test’s bias in multiple tree tests (Shimodaira and Hasegawa, 1999
P. glabra.P2, while these were rejected in the AU test but not in the K-H test. The S-H test showed very conservative behavior, as only 6 of 21 constraints were rejected, compared to the rejection of all constraints by the AU test, and 19 of 21 constraints by the K-H test (Table 3). Most of the conflicts, involving 23 species of the 40 species examined, appear to be statistically significant in both parsimony and likelihood-based tests (Tables 2 and 3). Interestingly, results of the S-H tests differed in suggesting fewer strong conflicts. The S-H test was proposed to compensate for the K-H test’s bias in multiple tree tests (Shimodaira and Hasegawa, 1999
Hybridization as a cause of tree incongruence
Our analyses support nonstochastic processes underlying the observed conflicts. It is difficult, however, to distinguish between underlying processes—horizontal gene transfer, incomplete lineage sorting, or hybridization. Although we cannot totally rule out horizontal transfer or lineage sorting, we favor hybridization as the main cause of the conflicts in Eupersicaria. Horizontal transfer is least likely, perhaps, because we know of no special mechanisms of transfer (viral or bacterial vectors or parasitism) in this group of plants (Richardson and Palmer, 2007![]() ). It is much more difficult to distinguish between incomplete lineage sorting and hybridization because these processes can produce almost identical outputs at the level of tree discordances (Rosenberg, 2002
). It is much more difficult to distinguish between incomplete lineage sorting and hybridization because these processes can produce almost identical outputs at the level of tree discordances (Rosenberg, 2002![]() ; Rokas et al., 2003
; Rokas et al., 2003![]() ; Doyle et al., 2004
; Doyle et al., 2004![]() ; Linder and Rieseberg, 2004
; Linder and Rieseberg, 2004![]() ). However, on the basis of several lines of evidence discussed later, we suggest that hybridization is the more likely process in this case.
). However, on the basis of several lines of evidence discussed later, we suggest that hybridization is the more likely process in this case.
Aside from the strong incongruence documented here between maternally inherited cpDNA and biparentally inherited nrITS, the most important evidence in support of hybridization is that many of the species involved in strong conflicts are polyploids. Excluding five known diploid species (P. hirsuta, P. setaceae, P. orientalis, P. viscofera, and P. viscosa), 10 of the 16 conflicting species are polyploids (Kim, 2008![]() ); chromosome numbers for the remaining six have not been reported. We suggest (further discussed later) that the tetra- and hexaploid Eupersicaria species involved in tree incongruence are allopolyploids stemming from hybridization, as proposed for many other groups, e.g., Cardamine (Lihova et al., 2006
); chromosome numbers for the remaining six have not been reported. We suggest (further discussed later) that the tetra- and hexaploid Eupersicaria species involved in tree incongruence are allopolyploids stemming from hybridization, as proposed for many other groups, e.g., Cardamine (Lihova et al., 2006![]() ), Glycine (Doyle et al., 2004
), Glycine (Doyle et al., 2004![]() ), Tragopogon (Soltis et al., 2004
), Tragopogon (Soltis et al., 2004![]() ), and Triticeae (Kellogg et al., 1996
), and Triticeae (Kellogg et al., 1996![]() ).
).
Additional evidence in favor of hybridization as the cause comes from previous studies in Eupersicaria. Plants in Eupersicaria are notoriously polymorphic in morphology, which has been attributed to hybridization (Stanford, 1925b![]() , 1926
, 1926![]() , 1927
, 1927![]() ; Fernald, 1950
; Fernald, 1950![]() ). More importantly, artificial crosses have been successful among several North American species at the same ploidal level (e.g., between diploid P. hirsuta and P. setacea and between tetraploid P. hydropiperoides and P. opelousana; McDonald, 1980
). More importantly, artificial crosses have been successful among several North American species at the same ploidal level (e.g., between diploid P. hirsuta and P. setacea and between tetraploid P. hydropiperoides and P. opelousana; McDonald, 1980![]() ). These experiments are critical in demonstrating the potential for hybridization (incomplete reproductive isolation) in plants that are quite often cleistogamous (Stanford, 1925a
). These experiments are critical in demonstrating the potential for hybridization (incomplete reproductive isolation) in plants that are quite often cleistogamous (Stanford, 1925a![]() ; Simmonds, 1945a
; Simmonds, 1945a![]() ). Finally, Consaul et al. (1991)
). Finally, Consaul et al. (1991)![]() examined isozyme profiles in the P. lapathifolia complex and proposed a hybrid origin for P. maculosa. Specifically, they suggested that the tetraploid P. maculosa was an allopolyploid species having the diploid P. lapathifolia as one possible parent. Interestingly, this particular origin is not evident in our tree incongruence results. That is, the placement of P. maculosa does not strongly conflict between the cpDNA and nrITS trees (Fig. 1). If P. maculosa is of hybrid origin, there must have been concerted evolution in P. maculosa nrITS sequences toward the maternal source lineage. In general, this homogenization in nrITS sequences will lead to an underestimation of hybridization events. Other approaches, such as the use of low copy nuclear genes, are needed to reveal such cases.
examined isozyme profiles in the P. lapathifolia complex and proposed a hybrid origin for P. maculosa. Specifically, they suggested that the tetraploid P. maculosa was an allopolyploid species having the diploid P. lapathifolia as one possible parent. Interestingly, this particular origin is not evident in our tree incongruence results. That is, the placement of P. maculosa does not strongly conflict between the cpDNA and nrITS trees (Fig. 1). If P. maculosa is of hybrid origin, there must have been concerted evolution in P. maculosa nrITS sequences toward the maternal source lineage. In general, this homogenization in nrITS sequences will lead to an underestimation of hybridization events. Other approaches, such as the use of low copy nuclear genes, are needed to reveal such cases.
Particular cases of allopolyploid speciation in Eupersicaria
Persicaria punctata likely originated from hybridization between the diploid species P. hydropiper and either P. hirsuta or P. setacea. This is clearly supported by the nrITS polymorphism; P. punctata had two different types of nrITS sequence, and each type was shared with a form in an inferred diploid parental species. More specifically, P. hydropiper may have provided the paternal parent based on the nrITS tree, and P. hirsuta or P. setacea may have served as the maternal parent based on the cpDNA tree if we consider allopolyploid speciation involving only known diploid parents (Figs. 1 and 2). Distingushing between P. hirsuta and P. setacea is difficult because these appear to be very closely related (Figs. 1 and 2). Persicaria hirsuta can be distinguished from P. setacea by hairs throughout the plant and more or less pink-colored perianth, but the two are very similar in other morphological traits. Experimental crosses between the two species showed that fully fertile seeds in the F1 progeny were produced only when P. hirsuta was used as the maternal lineage (McDonald, 1980![]() ). Morphologically, P. punctata shows intermediacy between P. hydropiper and P. hirsuta/setacea. Distinct glands on the perianth, peppery taste, and relatively glabrous stems and leaves in P. punctata are shared with P. hydropiper. On the other hand, P. punctata is similar to P. hirsuta/setacea in having relatively distinct and long inflorescences.
). Morphologically, P. punctata shows intermediacy between P. hydropiper and P. hirsuta/setacea. Distinct glands on the perianth, peppery taste, and relatively glabrous stems and leaves in P. punctata are shared with P. hydropiper. On the other hand, P. punctata is similar to P. hirsuta/setacea in having relatively distinct and long inflorescences.
Our results might also be consistent with a different interpretation of the origin of P. punctata given the support values in our cpDNA tree. Persicaria punctata belongs to a clade with P. hydropiperoides, P. opelousana, P. puritanorum, and P. robustior, with little resolution among them (Figs. 1A and 2A). It is possible, therefore, that another tetraploid species, P. hydropiperoides or P. opelousana, provided the maternal parent for P. punctata, but only if P. hydropiper supplied an unreduced paternal gamete (Appendix S2, see Supplemental Data with online version of article). The P. hydropiperoides complex itself, including P. hydropiperoides and P. opelousana, may also have originated via allopolyploid speciation. The diploid maternal parent most likely belonged to the P. hirsuta/setacea lineage, while the paternal parent is unclear. In the nrITS tree, the clade including the P. hydropiperoides complex and P. puritanorum is not directly linked with any diploid lineage.
The hexaploid P. densiflora is most closely related to the P. hydropiperoides complex in the nrITS tree, whereas it is clustered with the clade that includes P. lapathifolia in the cDNA tree (Figs. 1 and 2). On this basis, P. densiflora may have originated by a cross between a tetraploid paternal parent from the P. hydropiperoides complex or an unknown diploid species and a diploid maternal parent among P. lapathifloia, P. viscosa, or P. orientalis. Because P. viscosa and P. orientalis were originally distributed in Asia and are densely hairy throughout, the widely distributed P. lapathifolia, which is usually glabrous except for glandular trichomes in the inflorescence, is perhaps a more likely diploid parent for P. densiflora, which is glabrous and distributed in the New World.
The tetraploid P. minor, which is sister to the P. hydropiperoides complex and P. densiflora in the nrITS tree, may have arisen through hybridization between an unknown diploid lineage or possibly a tetraploid in the P. hydropiperoides complex and the diploid P. hydropiper. It is not entirely clear that P. hydropiper served as the maternal parent, however, because the relationship between P. hydropiper and P. minor is somewhat weakly supported in the cpDNA tree (Figs. 1A and 2A). Morphologically, P. minor is more similar to the diploid P. foliosa, which is nested in a clade including P. macrantha  P. maculosa and other tetraploid species (Fig. 1A). Inasmuch as the P. macrantha
P. maculosa and other tetraploid species (Fig. 1A). Inasmuch as the P. macrantha  P. maculosa clade (Fig. 1A) is closely related to the clade including P. hydropiper and P. minor (Fig. 1A), P. foliosa should also be considered as a possible diploid maternal lineage for P. minor.
P. maculosa clade (Fig. 1A) is closely related to the clade including P. hydropiper and P. minor (Fig. 1A), P. foliosa should also be considered as a possible diploid maternal lineage for P. minor.
Another possible allopolyploid species is P. nodosa. Persicaria nodosa has generally been considered a synonym of the diploid P. lapathifolia based on morphology (Mitchell and Dean, 1978![]() ; Gleason and Cronquist, 1991
; Gleason and Cronquist, 1991![]() ; Yang and Wang, 1991
; Yang and Wang, 1991![]() ; Hinds and Freeman, 2005
; Hinds and Freeman, 2005![]() ), but our nrITS sequence data indicate that P. nodosa is distinct from P. lapathifolia and instead is closely related to the diploid P. viscofera (Figs. 1 and 2). This observation, combined with our confirmation that P. nodosa is a tetraploid, leads us to hypothesize that P. nodosa originated via hybridization between P. lapathifolia and P. viscofera. However, this scenario raises a question regarding geographic distributions because P. viscofera populations are confined to East Asia, whereas P. nodosa populations are found in Europe and North America (Danser, 1921
), but our nrITS sequence data indicate that P. nodosa is distinct from P. lapathifolia and instead is closely related to the diploid P. viscofera (Figs. 1 and 2). This observation, combined with our confirmation that P. nodosa is a tetraploid, leads us to hypothesize that P. nodosa originated via hybridization between P. lapathifolia and P. viscofera. However, this scenario raises a question regarding geographic distributions because P. viscofera populations are confined to East Asia, whereas P. nodosa populations are found in Europe and North America (Danser, 1921![]() ; Simmonds, 1945b
; Simmonds, 1945b![]() ). Perhaps P. nodosa moved into Europe and North America following allopolyploid speciation in East Asia between the widely distributed P. lapathifolia and the East Asian P. viscofera (cf. Sang et al., 1997
). Perhaps P. nodosa moved into Europe and North America following allopolyploid speciation in East Asia between the widely distributed P. lapathifolia and the East Asian P. viscofera (cf. Sang et al., 1997![]() ).
).
It is difficult to evaluate the possibility of allopolyploid speciation for taxa whose chromosome numbers have not yet been determined. Chromosome numbers for three species (P. mexicana, P. segetum, and P. bicornis) have not been reported. Although the chromosome number for P. pensylvanica has been reported as 2n = 22 (Löve and Löve, 1982![]() ) or 2n =
) or 2n =  80 (Gervais, 2000
80 (Gervais, 2000![]() ), our preliminary count indicates that this species is possibly an octaploid. These four species form a strongly supported clade in the nrITS tree and in turn are linked with the clade that includes the diploid species P. hirsuta and P. setacea. Their paternal contribution may therefore have come from this lineage. The maternal contribution is unclear based on lack of resolution in the cpDNA tree (Figs. 1 and 2).
), our preliminary count indicates that this species is possibly an octaploid. These four species form a strongly supported clade in the nrITS tree and in turn are linked with the clade that includes the diploid species P. hirsuta and P. setacea. Their paternal contribution may therefore have come from this lineage. The maternal contribution is unclear based on lack of resolution in the cpDNA tree (Figs. 1 and 2).
Implications for relationships and taxonomy in Eupersicaria
In view of the strong disagreements between trees inferred from the nrITS and the combined cpDNA sequences and our tests indicating that these are mostly not due to stochastic error, we have not combined these data sets and will resist the temptation to discuss phylogenetic relationships within Eupersicaria. Instead, in this section we discuss several specific cases that bear on taxonomic treatments. In general, our analyses support the view that the number of species has been underestimated in polyploidy complexes (Soltis et al., 2007![]() ).
).
On the basis of a partial sampling of North American species, we proposed the resurrection of the New England endemic, P. puritanorum, which had frequently been synonymized with P. maculosa. Persicaria puritanorum, a hexaploid, does not appear to be closely related to the tetraploid P. maculosa, but instead likely originated via hybridization involving at least one parent from the P. hydropiperoides complex (Kim, 2008![]() ).
).
The circumscription of P. lapathifolia has caused difficulty. Timson (1963)![]() , following up on cultivation experiments by Danser (1921)
, following up on cultivation experiments by Danser (1921)![]() , delimited 12 lineages within the P. lapathifolia complex based on several morphological characters. He and many authors since (Mitchell and Dean, 1978
, delimited 12 lineages within the P. lapathifolia complex based on several morphological characters. He and many authors since (Mitchell and Dean, 1978![]() ; Gleason and Cronquist, 1991
; Gleason and Cronquist, 1991![]() ; Yang and Wang, 1991
; Yang and Wang, 1991![]() ; Hinds and Freeman, 2005
; Hinds and Freeman, 2005![]() ) have recognized P. lapathifolia in the broad sense, thus synonymizing P. nodosa, P. tomentosa, and others. Our analyses strongly support the resurrection of P. nodosa, which is a tetraploid (unpublished data) that probably originated through allopolyploid speciation, as suggested in earlier taxonomic treatments (Meisner, 1856
) have recognized P. lapathifolia in the broad sense, thus synonymizing P. nodosa, P. tomentosa, and others. Our analyses strongly support the resurrection of P. nodosa, which is a tetraploid (unpublished data) that probably originated through allopolyploid speciation, as suggested in earlier taxonomic treatments (Meisner, 1856![]() ; Danser, 1921
; Danser, 1921![]() ; Steward, 1930
; Steward, 1930![]() ; Britton, 1933
; Britton, 1933![]() ). Persicaria nodosa can be distinguished from the typical P. lapathifolia by red spots on the stems, the red-purple perianth near the base, and more or less coarse trichomes covering the whole plant (cf. Simmonds, 1945c
). Persicaria nodosa can be distinguished from the typical P. lapathifolia by red spots on the stems, the red-purple perianth near the base, and more or less coarse trichomes covering the whole plant (cf. Simmonds, 1945c![]() ).
).
Surprisingly, our incongruence tests indicated that differences in the placement of P. tomentosa (Figs. 1 and 2) might be due to stochastic error (Table 3: I, p; Table 4: viii), which may support the view that it represents a phenotypic extreme within P. lapathifolia (Danser, 1921![]() ; Timson, 1963
; Timson, 1963![]() ; Mitchell and Dean, 1978
; Mitchell and Dean, 1978![]() ; Gleason and Cronquist, 1991
; Gleason and Cronquist, 1991![]() ; Yang and Wang, 1991
; Yang and Wang, 1991![]() ; Hinds and Freeman, 2005
; Hinds and Freeman, 2005![]() ). However, we favor the recognition of P. tomentosa as a separate species on the basis of our observation that it is a tetraploid (S.-T. Kim, unpublished data) and possibly of allopolyploid origin and that it consistently has dense hairs on the undersides of the leaves. Confirming the allopolyploid origin in this group clearly requires additional work, including the use of low copy nuclear markers and further morphological analyses. The use of population genetic approaches, sampling from multiple populations, would also shed light on this issue.
). However, we favor the recognition of P. tomentosa as a separate species on the basis of our observation that it is a tetraploid (S.-T. Kim, unpublished data) and possibly of allopolyploid origin and that it consistently has dense hairs on the undersides of the leaves. Confirming the allopolyploid origin in this group clearly requires additional work, including the use of low copy nuclear markers and further morphological analyses. The use of population genetic approaches, sampling from multiple populations, would also shed light on this issue.
Another case in need of attention relates to Persicaria macrantha and P. japonica. Meisner (1856)![]() originally described these two species (as Polygonum macranthum and Polygonum japonicum), contrasting glandular dots on the perianth and larger flowers in the former with the lack of glands on the perianth and relatively small and more flowers in the later. Later, Nakai (1908)
originally described these two species (as Polygonum macranthum and Polygonum japonicum), contrasting glandular dots on the perianth and larger flowers in the former with the lack of glands on the perianth and relatively small and more flowers in the later. Later, Nakai (1908)![]() proposed the recognition of two varieties in P. japonica, one representing Meisner’s P. macrantha and the other representing Meisner’s P. japonica but with glands on the perianth. Steward (1930)
proposed the recognition of two varieties in P. japonica, one representing Meisner’s P. macrantha and the other representing Meisner’s P. japonica but with glands on the perianth. Steward (1930)![]() restored P. macrantha and P. japonica, but named a new variety in P. macrantha with glands on the perianth. In the most recent taxonomic treatment, Li et al. (2003)
restored P. macrantha and P. japonica, but named a new variety in P. macrantha with glands on the perianth. In the most recent taxonomic treatment, Li et al. (2003)![]() recognized two varieties in P. japonica. Our analyses strongly indicate that P. macrantha and P. japonica are distinct lineages (Figs. 1 and 2). It seems apparent from previous studies that there may be three possible entities, corresponding to P. macrantha, P. japonica with glands, and P. japonica without glands. The sample of P. japonica used in this study has glandular dots on the perianth. Further evaluation will require the inclusion of P. japonica individuals without these glands. In any case, however, our results strongly support the resurrection of P. macrantha.
recognized two varieties in P. japonica. Our analyses strongly indicate that P. macrantha and P. japonica are distinct lineages (Figs. 1 and 2). It seems apparent from previous studies that there may be three possible entities, corresponding to P. macrantha, P. japonica with glands, and P. japonica without glands. The sample of P. japonica used in this study has glandular dots on the perianth. Further evaluation will require the inclusion of P. japonica individuals without these glands. In any case, however, our results strongly support the resurrection of P. macrantha.
Lastly, we suggest that P. densiflora be treated as a distinct species, which possibly also originated via allopolyploidy. Persicaria densiflora, described by Meisner (1856)![]() based on American plants, has been merged into P. glabra mainly based on their sharing a glabrous plant body (Wilson, 1990
based on American plants, has been merged into P. glabra mainly based on their sharing a glabrous plant body (Wilson, 1990![]() ; Li et al., 2003
; Li et al., 2003![]() ; Hinds and Freeman, 2005
; Hinds and Freeman, 2005![]() ). Our analyses support three independent lineages based on the separation in our trees of P. densiflora from North America, and of the accessions of P. glabra from India and from Bolivia (Figs. 1 and 2). The morphological resemblances among these three lineages could reflect shared parentage in their allopolyploid origins or the convergence in loss of trichomes, more or less glandular dots on the perianth, and relatively longer petioles.
). Our analyses support three independent lineages based on the separation in our trees of P. densiflora from North America, and of the accessions of P. glabra from India and from Bolivia (Figs. 1 and 2). The morphological resemblances among these three lineages could reflect shared parentage in their allopolyploid origins or the convergence in loss of trichomes, more or less glandular dots on the perianth, and relatively longer petioles.
Summary
The strong conflicts we have shown between cpDNA and nrITS, combined with information on chromosome numbers and geography, support the view that there has been extensive hybridization and allopolyploid speciation in Eupersicaria. Specifically, our studies have identified 23 cases of conflict between nrITS and the combined cpDNA gene trees. We propose allopolyploidy as the mechanism underlying 10 known polyploid species of the 16 cases involving known polyploids that appear to be statistically meaningful in our congruence tests (Fig. 2). We cannot completely rule out the possibility that we have overestimated the number of allopolyploid events because other mechanisms can underlie discordance, but the concordance of different lines of evidence favors our interpretation. In fact, we are very likely to have underestimated allopolyploidy owing to limitations on the sample examined here and especially to concerted evolution in the nrITS. This problem is most evident in our finding nrITS polymorphism within P. punctata.
Additional studies are needed, employing different approaches (e.g., low copy nuclear markers; Sang, 2002![]() ; Įlvarez and Wendel, 2003
; Įlvarez and Wendel, 2003![]() ; Small et al., 2004
; Small et al., 2004![]() ), and with a focus on geographic and ecological patterns and the origin and spread of the important weedy species in this group. In the meantime, however, it now seems clear that multiple instances of hybridization and allopolyploidy have occurred in this group and that this has contributed to the taxonomic difficulties. It also appears that much of the diversification of this group has been driven by separate events of hybridization among diploids and allotetraploidy, as opposed to speciation following allotetraploidy (which would have resulted in clades of polyploid species). Finally, because many of these events are likely to have taken place relatively recently, more targeted comparisons in this group may be especially useful in elucidating the initial molecular and ecological impacts of allopolploidy (Paun et al., 2007
), and with a focus on geographic and ecological patterns and the origin and spread of the important weedy species in this group. In the meantime, however, it now seems clear that multiple instances of hybridization and allopolyploidy have occurred in this group and that this has contributed to the taxonomic difficulties. It also appears that much of the diversification of this group has been driven by separate events of hybridization among diploids and allotetraploidy, as opposed to speciation following allotetraploidy (which would have resulted in clades of polyploid species). Finally, because many of these events are likely to have taken place relatively recently, more targeted comparisons in this group may be especially useful in elucidating the initial molecular and ecological impacts of allopolploidy (Paun et al., 2007![]() ).
).
Appendix 1. Voucher information and GenBank accession numbers for taxa used in this study. Voucher specimens are deposited in the following herbaria: YU = Yale University, GH = Gray Herbarium, Harvard University, NHA = University of New Hampshire.
|
|
FOOTNOTES
1 The authors thank members of the Donoghue laboratory at Yale and S. Sultan of Wesleyan University for valuable comments and discussion, and C.-L. Ma, M. Deng, and M.-H. Kim for great help during fieldwork in China and Korea. They are indebted to GH, NHA, and YU for critical specimens and especially to N. Ritter for his collections and information on South American species. This work was partially supported by a John F. Enders Fellowship from Yale University. ![]()
2 Corresponding author (e-mail: sang-tae.kim@tuebingen.mpg.de); current address: Max Planck Institute for Developmental Biology, Department of Molecular Biology, Spemannstrasse 37-39, 72070, Tuebingen, Germany ![]()
LITERATURE CITED
Abbott, R. J., AND A. J. Lowe. 2004. Origins, establishment and evolution of new polyploid species: Senecio cambrensis and S. eboracensis in the British Isles. Biological Journal of the Linnean Society 82: 467–474.[CrossRef][ISI]
Įlvarez, I., AND J. F. Wendel. 2003. Ribosomal ITS sequences and plant phylogenetic inference. Molecular Biology and Evolution 29: 417–434.
Arnold, M. L. 1997. Natural hybridization and evolution. Oxford University Press, New York, New York, USA.
Arnold, M. L. 2006. Evolution through genetic exchange. Oxford University Press. New York, New York, USA.
Baum, D. A., R. L. Small, AND J. F. Wendel. 1998. Biogeography and floral evolution of baobabs (Adansonia, Bombacaceae) as inferred from multiple data sets. Systematic Biology 47: 181–207.[CrossRef][ISI][Medline]
Bell, D. L., AND S. E. Sultan. 1999. Dynamic phenotypic plasticity for root growth in Polygonum: A comparative study. American Journal of Botany 86: 807–819.
Britton, C. E. 1933. British polygona, section Persicaria. Journal of Botany 71: 90–98.
Brysting, A. K., B. Oxelman, K. T. Huber, V. Moulton, AND C. Brochmann. 2007. Untangling complex histories of genome mergings in high polyploids. Systematic Biology 56: 467–476.[CrossRef][ISI][Medline]
Buckley, T. R. 2002. Model misspecification and probabilistic tests of topology: Evidence from empirical data sets. Systematic Biology 51: 509–523.[CrossRef][ISI][Medline]
Buckley, T. R., C. Simon, H. Shimodaira, AND
G. K. Chambers. 2001. Evaluating hypotheses on the origin and evolution
of the New Zealand alpine cicadas (Maoricicada) using
multiple-comparison tests of tree topology. Molecular Biology and Evolution 18: 223–234.
Comai, L. 2005. The advantages and disadvantages of being polyploid. Nature Reviews Genetics 6: 836–846.[CrossRef][ISI][Medline]
Consaul, L. L., S. I. Warwick, AND J. McNeill. 1991. Allozyme variation in the Polygonum lapathifolium complex. Canadian Journal of Botany 69: 2261–2270.[CrossRef]
Danser, B. H. 1921. Contribution a la systematique du Polygonum lapathifolium. Recueil des Travaux Botaniques Neerlandais 18: 9–210.
Darlu, P., AND G. Lecointre. 2002. When does the incongruence length difference test fail? Molecular Biology and Evolution 19: 432–437.
DeBry, R. W., AND R. G. Olmstead. 2000. A simulation study of reduced tree-search effort in bootstrap resampling analysis. Systematic Biology 49: 171–179.[CrossRef][ISI][Medline]
Dolphin, K. B. R. O. C. D. L., AND D. L. J. Quicke. 2000. Noise and incongruence: Interpreting results of the incongruence length different test. Molecular Phylogenetics and Evolution 17: 401–406.[CrossRef][ISI][Medline]
Doolittle, W. F., Y. Boucher, C. L. Nesbų, C. J. Douady, J. O. Andersson, AND A. J. Roger. 2003. How big is the iceberg of which organellar genes in nuclear genomes are but the tip? Philosophical Transactions of the Royal Society, B. Biological Sciences 358: 39–57.[CrossRef][Medline]
Dowton, M., AND A. D. Austin. 2002. Increased congruence does not necessarily indicate increased phylogenetic accuracy—The behavior of the incongruence length difference test in mixed-model analyses. Systematic Biology 51: 19–31.[CrossRef][ISI][Medline]
Doyle, J. J., J. L. Doyle, J. T. Rauscher, AND A. H. D. Brown. 2004. Diploid and polyploid reticulate evolution throughout the history of the perennial soybeans (Glycine subgenus Glycine). New Phytologist 161: 121–132.[CrossRef][ISI]
Edgar, R. C. 2004. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797.
Farris, J. S., M. Källersö, A. G. Kluge, AND C. Bult. 1994. Testing significance of incongruence. Cladistics 10: 315–319.[CrossRef][ISI]
Farris, J. S., M. Källersjö, A. G. Kluge, AND C. Bult. 1995. Constructing a significance test for incongruence. Systematic Biology 44: 570–572.[CrossRef][ISI]
Fassett, N. C. 1949. The variation of Polygonum punctatum. Brittonia 6: 369–393.[CrossRef]
Felsenstein, J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791.[CrossRef][ISI]
Felsenstein, J. 2004. Inferring phylogenies. Sinauer, Sunderland, Massachusetts, USA.
Fernald, M. L. 1950. Grays manual of botany. Van Nostrand, New York, New York, USA.
Gervais, C. 2000. Documentation chromosomique. Contribution no. 1. Ludoviciana 29.
Gleason, H. A., AND A. Cronquist. 1991. Manual of vascular plants of northeastern United States and adjacent Canada. New York Botanical Garden, Bronx, New York, USA.
Goldman, N., J. P. Anderson, AND A. G. Rodrigo. 2000. Likelihood-based tests of topologies in phylogenetics. Systematic Biology 49: 652–670.[CrossRef][ISI][Medline]
Grant, V. 1981. Plant speciation, 2nd ed. Columbia University Press, New York, New York, USA.
Greene, E. L. 1904. Leaflets of botanical observation and criticism. Washington, D.C. USA.
Gross, M. H. 1913. Remarques sur les Polygonées de l’Asie Orientale. Bulletin de l’Academie Internationale de Geographie Botanique 23: 7–32.
Haraldson, K. 1978. Anatomy and taxonomy in Polygonaceae subfam. Polygonoideae Meisn. emend. Jaretzky. Symbolae Botanicae Upsalienses 22: 1–95.
Hegarty, M. J., AND S. J. Hiscock. 2005. Hybrid speciation in plants: New insights from molecular studies. New Phytologist 165: 411–423.[CrossRef][ISI][Medline]
Hinds, H. R., AND C. C. Freeman. 2005. Persicaria. In Flora of North America Editorial Committee [eds.], Flora of North America North of Mexico, vol. 5, 574–594. Oxford University Press, New York, New York, USA.
Hipp, A. L., J. C. Hall, AND K. J. Sytsma. 2004. Congruence versus phylogenetic accuracy: Revisiting the incongruence length difference test. Systematic Biology 53: 81–89.[CrossRef][ISI][Medline]
Holder, M. T., J. A. Anderson, AND A. K. Holloway. 2001. Difficulties in detecting hybridization. Systematic Biology 50: 978–982.[CrossRef][ISI][Medline]
Huelsenbeck, J. P., AND F. Ronquist. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics (Oxford, England) 17: 754–755.[CrossRef]
Kellogg, E. A., R. Appels, AND R. J. Mason-Gamer. 1996. When genes tell different stories: The diploid genera of Triticeae (Gramineae). Systematic Botany 21: 321–347.[CrossRef][ISI]
Kim, S.-T. 2008. The role of allopolyploid speciation in the diversification of Persicaria (Polygonaceae). Ph.D. dissertation, Yale University, New Haven, Connecticut, USA.
Kim, S.-T., AND M. J. Donoghue. 2008. Molecular phylogeny of Persicaria (Polygonaceae). Systematic Botany 33: 77–86.[CrossRef][ISI]
Kishino, H., AND M. Hasegawa. 1989. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. Journal of Molecular Evolution 29: 170–179.[CrossRef][ISI][Medline]
Kishino, H., T. Miyata, AND M. Hasegawa. 1990. Maximum likelihood inference of protein phylogeny and the origin of chloroplasts. Journal of Molecular Evolution 31: 151–160.[CrossRef][ISI]
Lamb Frye, A. S., AND K. A. Kron. 2003. rbcL phylogeny and character evolution in Polygonaceae. Systematic Botany 28: 326–332.[ISI]
Lecointre, G., L. Rachdi, P. Darlu, AND E. Denamur. 1998. Escherichia coli molecular phylogeny using the incongruence length difference test. Molecular Biology and Evolution 15: 1685–1695.[Abstract]
Li, A., B. Bao, A. E. Grabovskaya-Borodina, S.-P. Hong, J. McNeill, S. L. Mosyakin, M. Ohba, AND C.-W. Park. 2003. Polygonaceae. In Z.-Y. Wu, and P. H. Raven [eds.], Flora of China, vol. 5, 277–350. Missouri Botanical Garden Press, St. Louis, Missouri, USA.
Lihova, J., K. K. Shimizu, AND K. Marhold. 2006. Allopolyploid origin of Cardamine asarifolia (Brassicaceae): Incongruence between plastid and nuclear ribosomal DNA sequences solved by a single-copy nuclear gene. Molecular Biology and Evolution 39: 759–786.
Linder, C. R., AND L. H. Rieseberg. 2004. Reconstructing patterns of reticulate evolution in plants. American Journal of Botany 91: 1700–1708.
Lis, J. T., AND R. Schleif. 1975. Size fractionation of double-stranded DNA by precipitation with polyethylene glycol. Nucleic Acids Research 2: 383–389.
Löve, A., AND D. Löve. 1982. IOPB chromosome number reports LXXIV. Taxon 31: 120–126.
Maddison, W. P. 1997. Gene trees in species trees. Systematic Biology 46: 523–536.[CrossRef][ISI]
Mason-Gamer, R. J., AND E. A. Kellogg. 1996. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545.[CrossRef][ISI]
McDonald, C. B. 1980. A biosystematic study of the Polygonum hydropiperoides (Polygonaceae) complex. American Journal of Botany 67: 664–670.[CrossRef][ISI]
Meisner, C. F. 1826. Monographiae generis polygoni prodromus. Sumtibus Auctoris, Typis A. Lador, Geneva, Switzerland.
Meisner, C. F. 1856. Polygonaceae. In A. de Candole [ed.], Prodromus systematis naturalis regni vegetabilis, 1–186, 693–695. Sumptibus Sociorum Treuttel et Wurtz, Paris, France.
Mitchell, R. S. 1968. Variation in the Polygonum amphibium complex and its taxonomic significance. University of California Publications in Botany 45: 1–65.
Mitchell, R. S. 1976. Submergence experiments on nine species of semi-aquatic Polygonum. American Journal of Botany 63: 1158–1165.[CrossRef][ISI]
Mitchell, R. S., AND J. K. Dean. 1978. Polygonaceae (buckwheat family) of New York State. New York State Museum Bulletin 431: 1–79.
Nakai, T. 1908. Polygonaceae koreanae. Journal of the College of Science. Imperial University of Tokyo. 23: 1–28.
Notredame, C., D. Higgins, AND J. Heringa. 2000. T-coffee: A novel method for multiple sequence alignments. Journal of Molecular Biology 302: 205–217.[CrossRef][ISI][Medline]
Otto, S. P., AND J. Whitton. 2000. Polyploid incidence and evolution. Annual Review of Genetics 34: 401–437.[CrossRef][ISI][Medline]
Paun, O., M. F. Fay, D. E. Soltis, AND M. W. Chase. 2007. Genetic and epigenetic alterations after hybridization and genome doubling. Taxon 56: 649–656.[ISI]
Planet, P. J. 2006. Tree disagreement: Measuring and testing incongruence in phylogenies. Journal of Biomedical Informatics 39: 86–102.[CrossRef][ISI][Medline]
Posada, D., AND K. A. Crandall. 1998. MODELTEST: Testing the model of DNA substitution. Bioinformatics (Oxford, England) 14: 817–818.[CrossRef]
Richardson, A. O., AND J. D. Palmer. 2007. Horizontal gene transfer in plants. Journal of Experimental Botany 148: 1–9.
Rieseberg, L. H. 1997. Hybrid origins of plant species. Annual Review of Ecology and Systematics 28: 359–389.[CrossRef][ISI]
Rieseberg, L. H., AND J. H. Willis. 2007. Plant speciation. Science 317: 910–914.
Rokas, A., G. Melika, Y. Abe, J.-L. Nieves-Aldrey, J. M. Cook, AND G. N. Stone. 2003. Lifecycle closure, lineage sorting, and hybridization revealed in a phylogenetic analysis of European oak gallwasps (Hymenoptera: Cynipidae: Cynipini) using mitochondrial sequence data. Molecular Phylogenetics and Evolution 26: 36–45.[CrossRef][ISI][Medline]
Rosenberg, N. A. 2002. The probability of topological concordance of gene trees and species trees. Theoretical Population Biology 61: 225–247.[CrossRef][ISI][Medline]
Sang, T. 2002. Utility of low-copy nuclear gene sequences in plant phylogenetics. Critical Reviews in Biochemistry and Molecular Biology 37: 121–147.[CrossRef][ISI][Medline]
Sang, T., D. J. Crawford, AND T. F. Stuessy. 1997. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia. American Journal of Botany 84: 1120–1136.[Abstract]
Shimodaira, H. 2002. An approximately unbiased test of phylogenetic tree selection. Systematic Biology 51: 492–508.[CrossRef][ISI][Medline]
Shimodaira, H., AND M. Hasegawa. 1999. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Molecular Biology and Evolution 16: 1114–1116.[ISI]
Shimodaira, H., AND M. Hasegawa. 2001. CONSEL: For assessing the confidence of phylogenetic tree selection. Bioinformatics (Oxford, England) 17: 1246–1247.[CrossRef]
Simmonds, N. W. 1945a. Polygonum persicaria L. Journal of Ecology 33: 121–131.[CrossRef][ISI]
Simmonds, N. W. 1945b. Polygonum lapathifolium L. (P. tomentosum Schr. of many continental authors). Journal of Ecology 33: 132–139.[CrossRef][ISI]
Simmonds, N. W. 1945c. Polygonum petecticale (Stokes) Druce (P. maculatum Trimen & Dyer, P. nodosum Persoon, etc.). Journal of Ecology 33: 140–143.[CrossRef][ISI]
Small, R. L., R. C. Cronn, AND J. F. Wendel. 2004. Use of nuclear genes for phylogeny reconstruction in plants. Australian Systematic Botany 17: 145–170.[CrossRef][ISI]
Soltis, D. E., AND P. S. Soltis. 1999. Polyploidy: Recurrent formation and genome evolution. Trends in Ecology & Evolution 14: 348–352.[CrossRef][ISI][Medline]
Soltis, D. E., P. S. Soltis, M. D. Bennett, AND I. J. Leitch. 2003. Evolution of genome size in the angiosperms. American Journal of Botany 90: 1596–1603.
Soltis, D. E., P. S. Soltis, J. C. Pires, A. Kovarik, J. A. Tate, AND E. Mavrodiev. 2004. Recent and recurrent polyploidy in Tragopogon (Asteraceae): Cytogenetic, genomic and genetic comparisons. Biological Journal of the Linnean Society 82: 485–501.[CrossRef][ISI]
Soltis, D. E., P. S. Soltis, D. W. Schemske, J. F. Hancock, J. N. Thompson, B. C. Husband, AND W. S. Judd. 2007. Autopolyploidy in angiosperms: Have we grossly underestimated the number of species? Taxon 56: 13–30.[ISI]
Stanford, E. E. 1925a. The inflorescence and flower-form in Polygonum, subgenus Persicaria. Rhodora 27: 41–47.
Stanford, E. E. 1925b. Possibilities of hybridism as a cause of variation in Polygonum. Rhodora 27: 81–89.
Stanford, E. E. 1926. Polygonum hydropiperoides and P. opelousanum. Rhodora 28: 11–17, 22-29.
Stanford, E. E. 1927. Polygonum hydropiper in Europe and North America. Rhodora 29: 77–87.
Stebbins, G. L. Jr. 1950. Variation and evolution in plants. Columbia University Press, New York, New York, USA.
Steward, A. N. 1930. The Polygonaceae of Eastern Asia. Contributions from the Gray Herbarium of Harvard University 5: 1–129.
Sultan, S. E. 2001. Phenotypic plasticity for fitness components in Polygonum species of contrasting ecological breadth. Ecology 82: 328–343.[CrossRef][ISI]
Sultan, S. E. 2003. Phenotypic plasticity in plants: A case study in ecological development. Evolution & Development 5: 25–33.[CrossRef][ISI][Medline]
Sultan, S. E., A. M. Wilczek, S. D. Hann, AND B. J. Brosi. 1998. Contrasting ecological breadth of co-occuring annual Polygonum species. Journal of Ecology 86: 363–383.[CrossRef][ISI]
Swofford, D. L. 2002. PAUP*: Phylogenetic analysis using parsimony (*and other methods). Sinauer, Sunderland, Massachusetts, USA.
Taberlet, P., L. Gielly, G. Pautou, AND J. Bouvet. 1991. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109.[CrossRef][ISI][Medline]
Templeton, A. R. 1983. Phylogenetic inference from restriction endonuclease cleavage site maps with particular reference to the evolution of humans and the apes. Evolution 37: 221–224.[CrossRef][ISI]
Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, AND
D. G. Higgins. 1997. The CLUSTAL_X windows interface: Flexible
strategies for multiple sequence alignment aided by quality analysis
tools. Nucleic Acids Research 25: 4876–4882.
Thornton, J. W., AND R. A. DeSalle. 2000. A new method to localize and test the significance of incongruence: Detecting domain shuffling in the nuclear receptor superfamily. Systematic Biology 49: 183–201.[CrossRef][ISI][Medline]
Timson, J. 1963. The taxonomy of Polygonum lapathifolium L. P. nodosum Pers. and P. tomentosum Schrank. Watsonia 5: 386–395.
Timson, J. 1964. A study of hybridization in Polygonum section Persicaria. Journal of Linnean Society (Botany) 59: 155–160.
White, T. J., T. D. Bruns, S. B. Lee, AND J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White [eds.], PCR protocols: A guide to methods and application, 315–322. Academic Press, San Diego, California, USA.
Wilson, K. L. 1990. Some widespread species of Persicaria (Polygonaceae) and their allies. Kew Bulletin 45: 621–636.[CrossRef]
Yang, J., AND J.-W. Wang. 1991. A taxometric analysis of characters of Polygonum lapathifolium L. Acta Phytotaxonomica Sinica 29: 258–263.
Yoder, A. D., J. A. Irwin, AND B. A. Payseur. 2001. Failure of the ILD to determine data combinability for slow loris phylogeny. Systematic Biology 50: 408–424.[ISI][Medline]
Young, N. D., K. E. Steiner, AND C. W. dePamphilis. 1999. The evolution of parasitism in Scrophulariaceae/Orobanchaceae: Plastid gene sequences refute an evolutionary transition series. Annals of the Missouri Botanical Garden 86: 876–893.[CrossRef][ISI]
Zelwer, M., AND V. Daubin. 2004. Detecting phylogenetic incongruence using BIONJ: An improvement of the ILD test. Molecular Phylogenetics and Evolution 33: 687–693.[CrossRef][ISI][Medline]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |